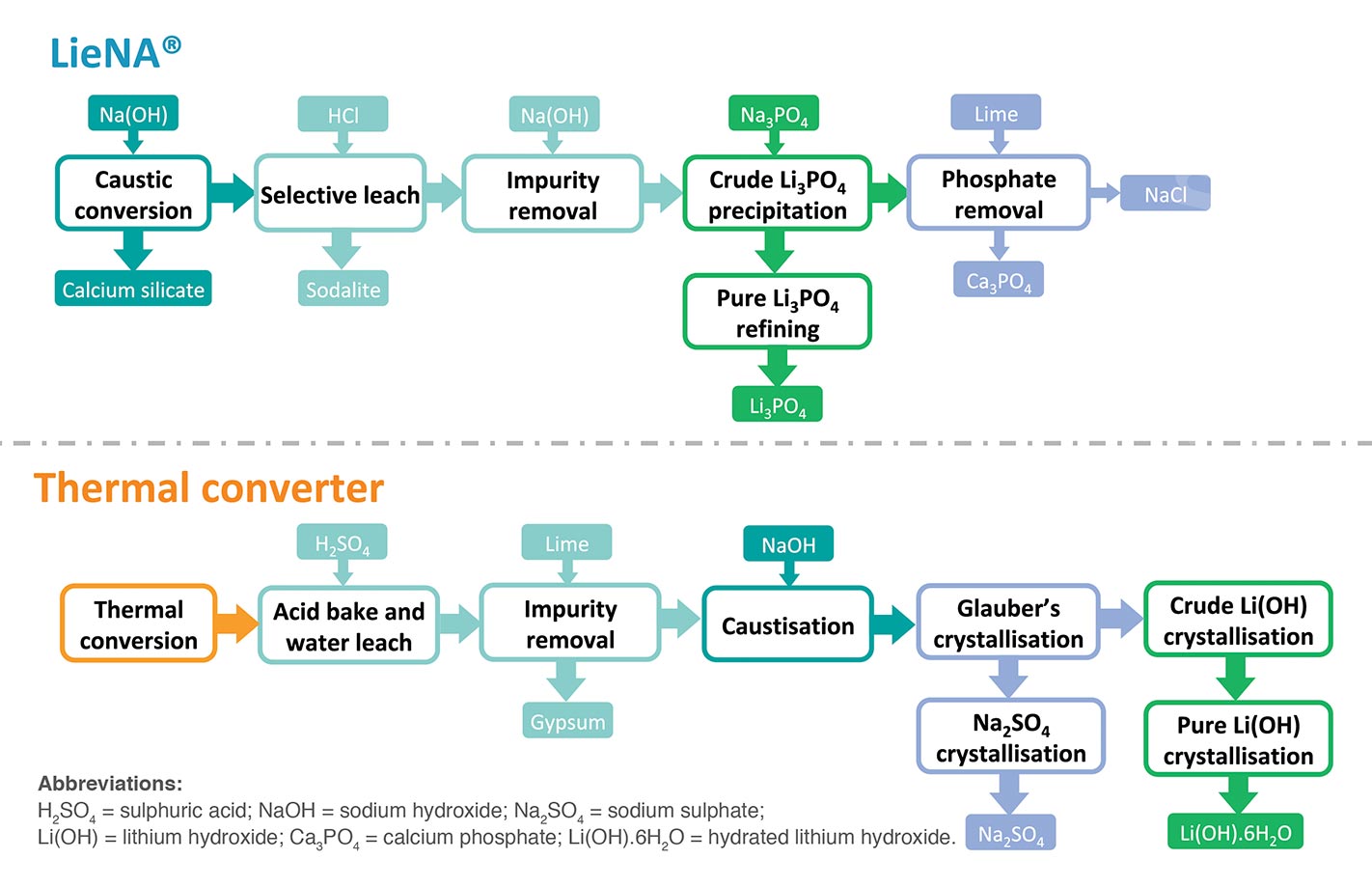
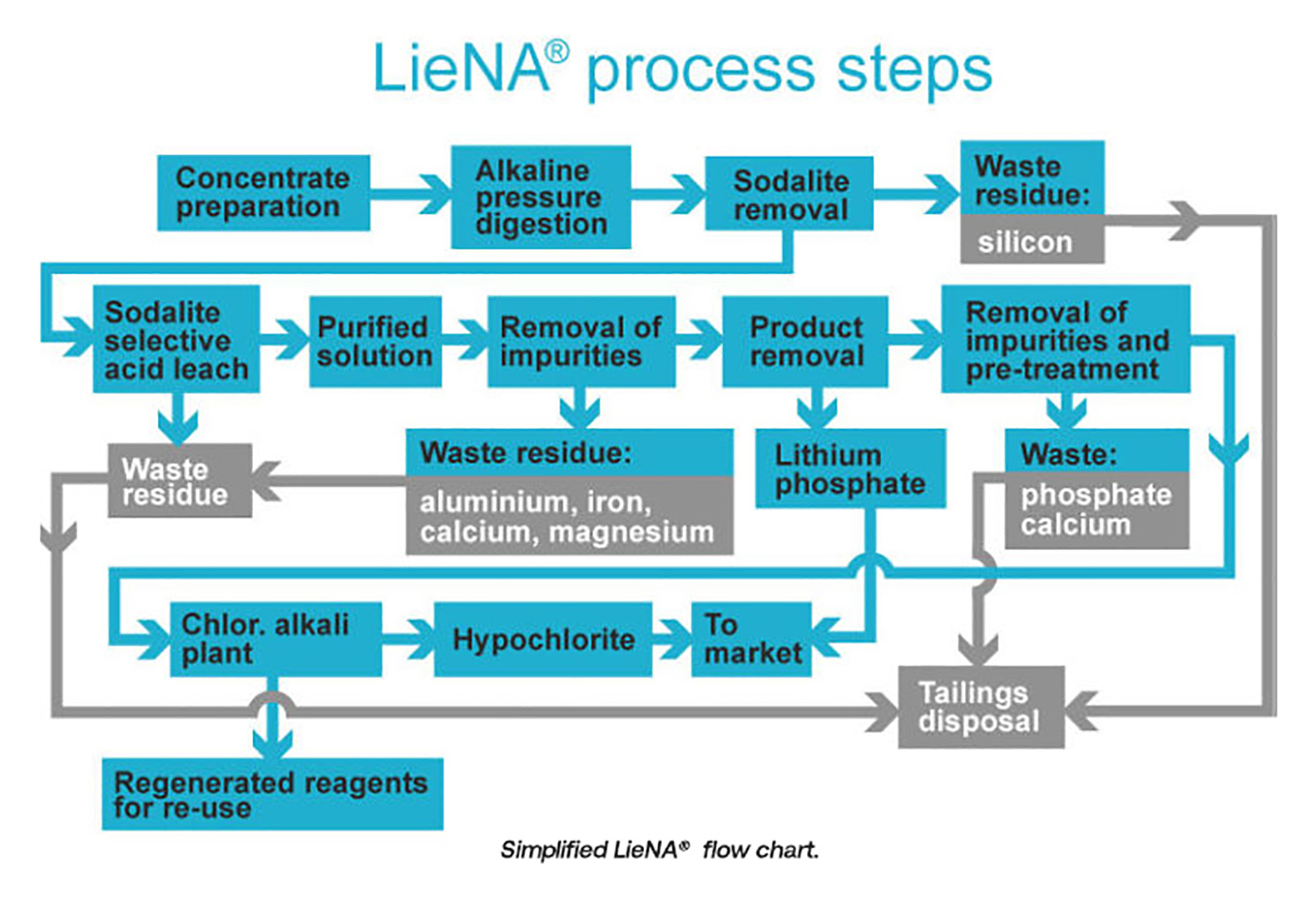
Lithium was first produced commercially in 1923. Aside from its use, historically, as a mood-stabilising medication, it’s also an efficient heat-transfer agent. Indeed, lithium and its compounds are intrinsic to numerous commercial applications, among them the manufacture of high-temperature lubricants, high strength-to-weight alloys (used, for example, in aircraft), heat-resistant glass and ceramics and, in the form of lithium hydroxide, as a means of removing carbon dioxide from the atmosphere of spacecraft.
In the 21st century, lithium’s electrochemical potential makes it a vital component of lithium-ion (‘Li-ion’) batteries. Li-ion batteries do have limitations in terms of cost, safety and stability; however, their energy-to-weight ratio, ability to be recharged many times over, and slow loss of charge when not in use have led three industry groups in particular – electric vehicles (‘EVs’), consumer electronics and the energy sector – to promote research and development into this battery technology.